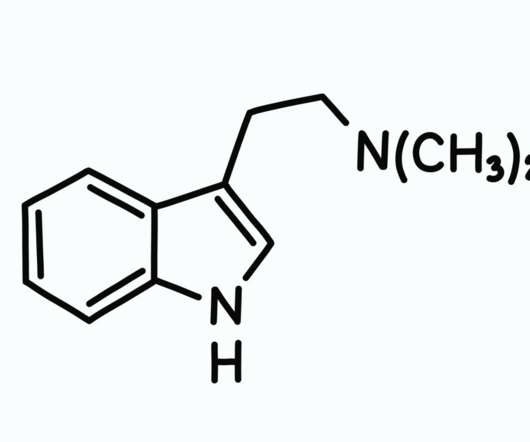
DEA Seeks to Increase Quota of Cannabis and Psilocybin for Research Purposes
SpeedWeed
SEPTEMBER 4, 2021
The DEA published a new document in the Federal Register on September 2 requesting an increase in production for certain Schedule I and Schedule II substances so that it can initiate more research studies. . DEA firmly believes in supporting regulated research of schedule I controlled substances. View original article.














































Let's personalize your content