New U.S. Research Efforts Could Catalyze Medical Cannabis’ Adoption in the Pharmaceutical Industry
Veriheal
APRIL 11, 2022
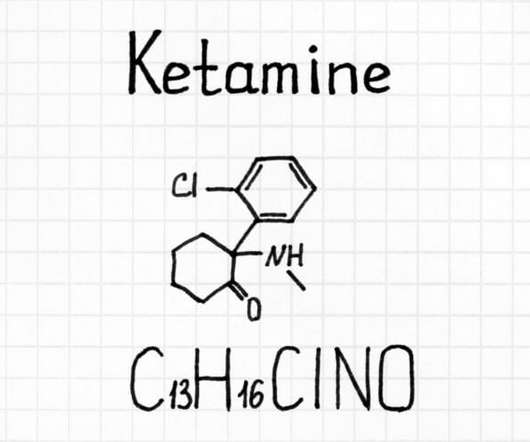
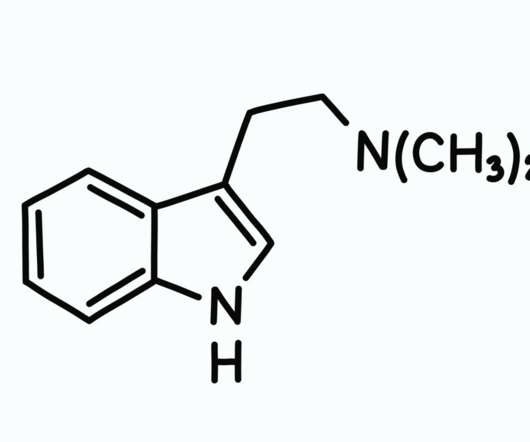
The final goal is to introduce medicinal cannabis products to the pharmaceutical drug market. He also emphasized the fact that medical cannabis programs are now legal in 37 states, as well as four out of five inhabited U.S. The company initiated the first legal transfer (as deemed by federal law) to the U.S. territories.













































Let's personalize your content