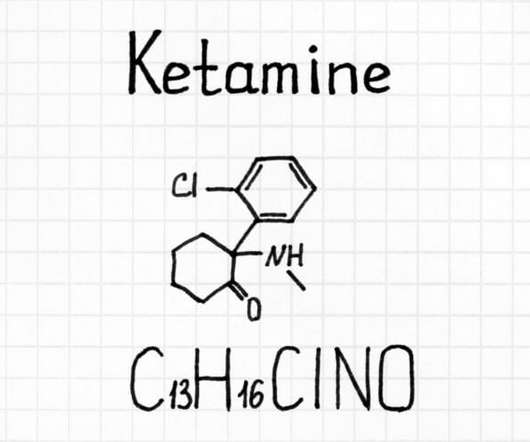
DEA-Licensed Biopharmaceutical Research Company to Partner with CURE Pharmaceutical to Produce Federally-Compliant Cannabis-Based Medical Products
Cannabis Law Report
JUNE 12, 2021
MONTEREY, CA – Biopharmaceutical Research Company (BRC), an active Drug Enforcement Administration (DEA) license holder, and CURE Pharmaceutical Holding Corp. CURE has established itself as a leader in the oral application of cannabis-derived treatments, and we know we’ll be able to bring a lot of value to patients together.”.









































Let's personalize your content