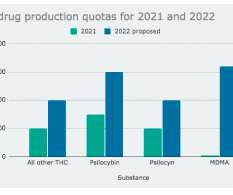
DEA Approves Cocaine Derivative for Parkinson’s Research. Why Not Cannabis?
Veriheal
JANUARY 24, 2023
As cannabis enthusiasts, we’re all too familiar with the stigma surrounding its use and the hoops that have to be jumped through to get it into medical research trials. But recent news of the DEA’s approval of a cocaine derivative for Parkinson’s disease research has left us scratching our heads. So what gives?















































Let's personalize your content