MDMA Failed to Receive FDA Approval: What Happens Next?
Veriheal
JUNE 26, 2025
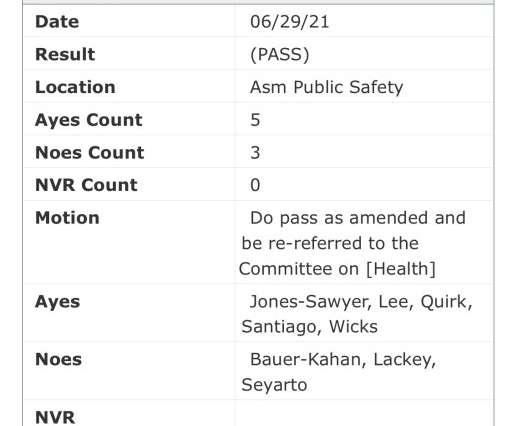
Drug company Lykos Therapeutics, which had filed the new drug application for MDMA therapy , was informed by the US Food and Drug Administration (FDA) that the data submitted was insufficient to receive approval, requesting that the company conduct an additional Phase 3 trial to further test the safety and efficacy of the drug.















































Let's personalize your content