DEA Approves Cocaine Derivative for Parkinson’s Research. Why Not Cannabis?
Veriheal
JANUARY 24, 2023
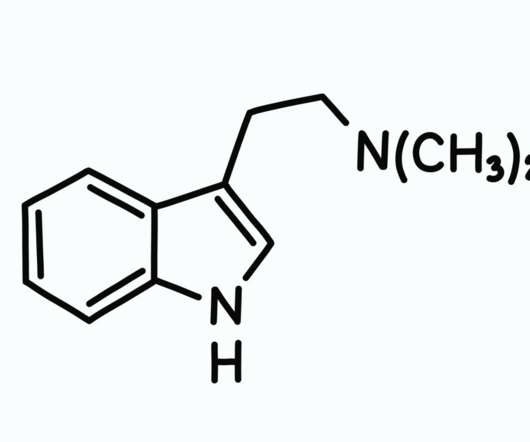
But recent news of the DEA’s approval of a cocaine derivative for Parkinson’s disease research has left us scratching our heads. After receiving a petition three years prior, the DEA finally answered with action, making plans to deschedule [18F]FP-CIT , a controlled substance derived from cocaine. .”














































Let's personalize your content