How Medical Marijuana is Cultivated, Processed, and Distributed in the USA
MMJ Recs
APRIL 30, 2024
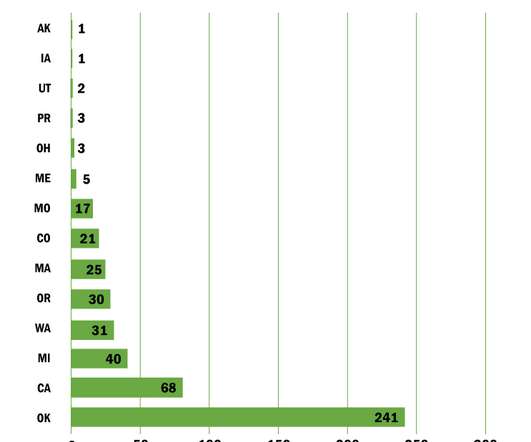
These regulations aim to maintain quality control, prevent diversion to the illicit market, and ensure patient safety. Cannabis extraction methods enable the isolation and concentration of specific compounds for use in medical marijuana products. Marijuana processing techniques vary depending on the desired end products.





































Let's personalize your content