The Tom Marino Roller Coaster: Three Days of Drug Policy Changes
Americans for Safe Access
OCTOBER 17, 2017
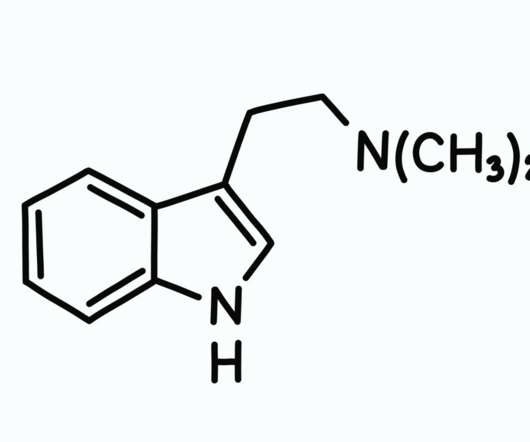
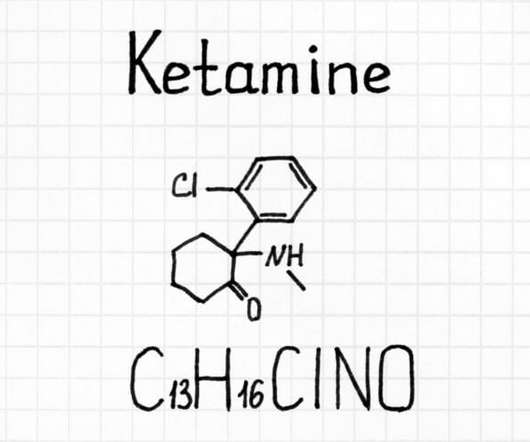
On October 15, 2017, the Washington Post and 60 Minutes released a scathing report on the pharmaceutical industry’s influence of the Drug Enforcement Administration (DEA).








































Let's personalize your content