Final Phase of MDMA Clinical Trials Shows Drug Is 90% Effective at Treating PTSD
SpeedWeed
MAY 15, 2021
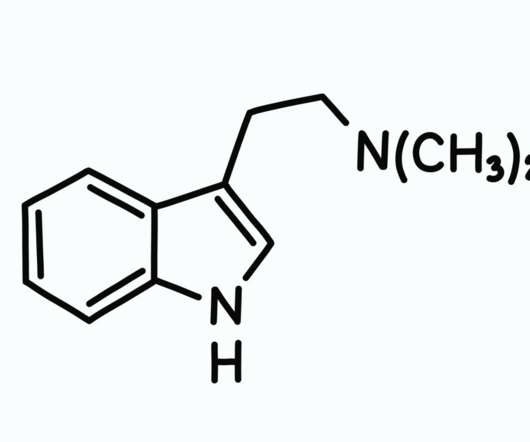
A first-of-its-kind Phase 3 clinical trial has found that MDMA-assisted therapy provided meaningful relief from PTSD symptoms in nearly 90 percent of study participants. MAPS is now enrolling participants for a further Phase 3 study that will allow another 50 subjects to access this promising new therapy. View original article.












































Let's personalize your content