Intoxicating Hemp Swindle
Project CBD
MARCH 7, 2025
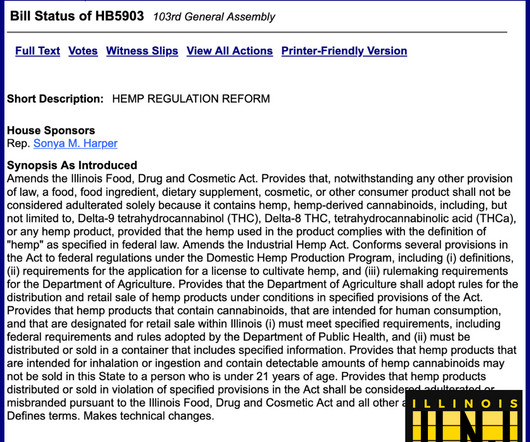
A new study from an industry advocacy group in California examines the content of dozens of unregulated intoxicating “hemp” products that are easily available in the Golden State despite being banned by state law. However, the following year the DEA put off its decision pending further public commentary. percent THC.











































Let's personalize your content