DEA Faces Lawsuit For Denying End of Life Patients the Right to Try Psychedelics Therapy
Veriheal
JUNE 29, 2021
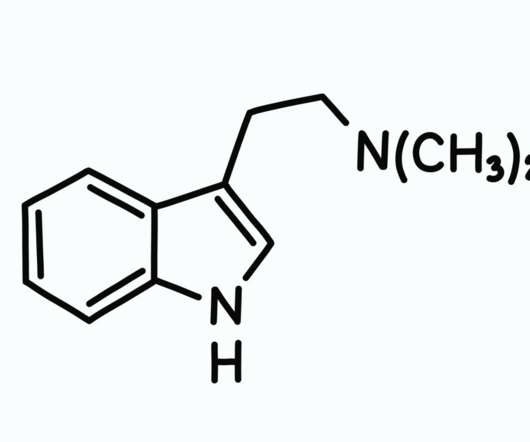
In order to legally access other therapies including nature-based options such as psilocybin , you must gain access under a Right to Try law which requires you to have a terminal condition. The lawsuit came after the DEA denied their application to utilize a synthetic form of psilocybin under the RTT laws.































Let's personalize your content