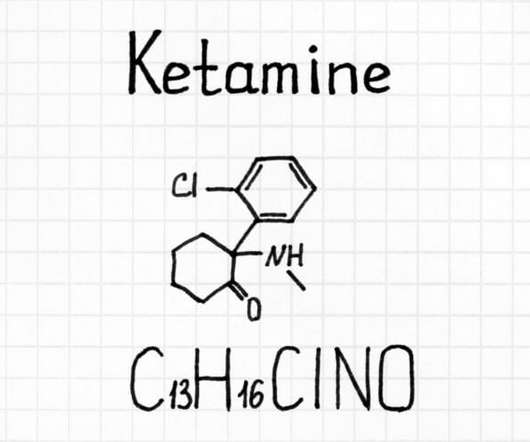
What is CBN (Cannabinol)? How does the natural byproduct of THC work?
The Cannigma
MARCH 7, 2022
What can the current clinical trials tell us? A common theme with cannabis is that more research is needed, so thankfully there are clinical trials in the works looking at the therapeutic utility of CBN. Those enrolled in the study will receive 28 days of treatment and then be assessed by pain-rating scales.














































Let's personalize your content