Perkins Coie: Prop 65 Plaintiffs Set Their Sights on Cannabis Industry
Cannabis Law Report
JULY 13, 2022
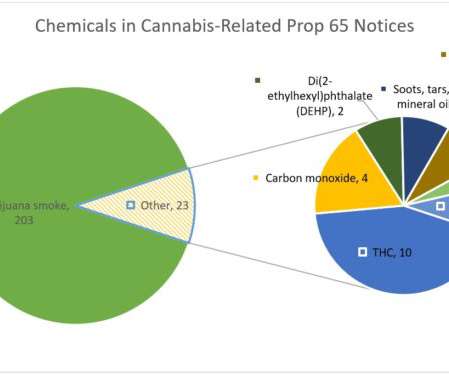
Over the past ten years, Proposition 65 plaintiffs have turned their sights on the cannabis industry, issuing over 200 notices of violation for a variety of chemicals in products from smokable marijuana flower to CBD oil, and vape pens to hemp-based personal care products.


















Let's personalize your content