DEA loosens rules on prescription CBD drug Epidiolex
Leafly
APRIL 6, 2020
Prescription CBD tincture Epidiolex is coming to more patients. The post DEA loosens rules on prescription CBD drug Epidiolex appeared first on Leafly. Cost: $32,500 per year.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

MJ Business Attorneys
JUNE 29, 2018
Epidiolex is the first drug the FDA has approved that contains a substance derived from marijuana, and it is also the first drug approved by the FDA to be used to treat patients with Dravet syndrome. Moreover, the ball is in the Drug Enforcement Agency’s (DEA) court. In the recent case Hemp Industries Associations v.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Veriheal
APRIL 11, 2022
The final goal is to introduce medicinal cannabis products to the pharmaceutical drug market. You don’t take that opium poppy and ask a patient to rub it on their lip for morphine. Drug Enforcement Agency (DEA). “We know cannabis has powerful potential medical effects. territories. This means that U.S.

Cannabis Cheri
DECEMBER 17, 2021
Locate a nurse and ask how many patients are in the respiratory wing due to their use of tobacco. You will learn that most of the patients in that wing are there for that reason. Now ask how many patients are there because they only smoked marijuana. This article is all about the effects of marijuana on the lungs.

Cannabis Law Report
JUNE 10, 2021
–(BUSINESS WIRE)– CURE Pharmaceutical Holding Corp. Currently, CURE uses its Schedule 1 DEA license for the development of Cannabis (THC) oral film products and other psychoactive substances, and manufactures multiple commercial Cannabidiol (CBD) products in compliance with the 2018 U.S. OXNARD, Calif.–(BUSINESS

Veriheal
JUNE 29, 2021
While they offer approved clinical trials that can offer patients the ability to try potential therapeutic options, many patients are unable to participate in clinical trials, and this is where the importance of the Right to Try programs comes into play. A Push by Patients. Patients Are Not In This Alone.

Cannabis Law Report
JUNE 12, 2021
MONTEREY, CA – Biopharmaceutical Research Company (BRC), an active Drug Enforcement Administration (DEA) license holder, and CURE Pharmaceutical Holding Corp. CURE has established itself as a leader in the oral application of cannabis-derived treatments, and we know we’ll be able to bring a lot of value to patients together.”.

Canna Law Blog
MARCH 4, 2020
People are increasingly using ketamine for ailments that resist treatment through traditional pharmaceutical drugs. “ Off-label use ” (which is extremely common) is: the use of pharmaceutical drugs for an unapproved indication or in an unapproved age group, dosage, or route of administration.

Veriheal
JUNE 22, 2021
While the federal government still holds medical cannabis or even recreational cannabis from the people clinging to years of draconian prohibition, they are allowing certain individuals to work with the DEA and FDA to study antimicrobial properties of cannabinoid therapies being applied to fighting bacteria such as MRSA.

Freedom Leaf
OCTOBER 15, 2018
On September 28, the DEA designated Epidiolex —a plant-based CBD pharmaceutical manufactured by the UK-based GW Pharmaceuticals—a Schedule V drug in the government’s list of controlled substances. schedules were created by the Controlled Substances Act (CSA) in 1970 and are interpreted and enforced by the DEA.

Cannabis Law Report
NOVEMBER 22, 2019
MMJ International Holdings, the premier medical cannabis research company, announced that it has received DEA approval to ship THC and CBD from Canada. “Patients will benefit from cGMP-quality therapies in an accessible and efficient format.”. .

Cannabis Cheri
DECEMBER 17, 2021
Locate a nurse and ask how many patients are in the respiratory wing due to their use of tobacco. You will learn that most of the patients in that wing are there for that reason. Now ask how many patients are there because they only smoked marijuana. This article is all about the effects of smoking marijuana and lung health.

CannaMD
AUGUST 8, 2020
Drugs, substances, and certain chemicals used to make drugs are classified into five categories (known as schedules ) depending on the Department of Drug Enforcement Agency (DEA)’s definition of the drug’s acceptable medical use and abuse/dependency potential. ”, our first instinct is to remain patients that CBD is CBD.

SpeedWeed
JUNE 15, 2021
The British cannabis pharmaceutical company takes home an award that demonstrates how important cannabis has been to the British economy for over 20 years—but also how much it so far has been reserved, as an industry, and as a drug, for the elite. GW Pharmaceuticals was just awarded a so-called “ Queen’s Prize for Innovation.” .

MedicalJane
MAY 14, 2020
As a result, research on the health effects of cannabis and cannabinoids has been limited in the United States, leaving patients, healthcare professionals, and policy makers without the necessary proof they need to make sound decisions regarding the use of cannabis as a medicine.

SpeedWeed
AUGUST 8, 2021
It’s the first cannabis-derived pharmaceutical to be legalized in many countries around the world, including the UK, Spain, United States, Canada, and New Zealand. . If all goes according to plan, patients will receive Sativex and the temozolomide chemotherapy drug, temozolomide on its own, or a placebo.

MedicalJane
JUNE 29, 2018
The new drug is indicated for the treatment of seizures associated with two rare and severe forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome, in patients two years of age and older. It is also the first FDA approval of a drug for the treatment of patients with Dravet syndrome. The Next Move Is On The DEA.

Cannabis Law Report
DECEMBER 15, 2021
The importation of the naturally derived psilocybin into California was completed after the Company’s supply partner Mycrodose Therapeutics was granted an import license from the United States Drug Enforcement Administration (DEA). “With their track-record of working with the U.S. . About Mycrodose Therapeutics.

NewsMunchies
SEPTEMBER 5, 2018
A new marijuana-derived drug to treat rare forms of severe epilepsy in children will cost about $32,500 per patient, per year. Epidiolex , the first cannabis pharmaceutical to be approved by the U.S. But at what cost to the patient or consumer is cannabis available or unavailable? We expect to make Epidiolex available to U.S.

Veriheal
DECEMBER 28, 2021
D espite the Drug Enforcement Agency’s (DEA) announcement in May that it would soon start reviewing grower applications for research purposes, cannabis research continues to be tightly restricted. For example, pharmaceutical-grade cannabis is being sold across the fast-evolving retail pharmacy landscape.

Cannabis Law Report
JULY 1, 2021
Things have changed a little in the past twenty years, when the DEA gave some researchers permission to study limited amounts of certain psychedelics. But they remain relatively small, in part because the DEA limits the amount of psilocybin and other psychedelics that can be produced each year. Who might be left behind?

SpeedWeed
MARCH 28, 2021
That’s what most providers are worried about when their patients with PTSD decide to try cannabis.”. As a result, there has been more interest among patients, clinicians, and researchers to see if cannabis is effective in treating PTSD. . Many veterans with PTSD already use cannabis to self-treat their symptoms.

Cannabis Law Report
JULY 9, 2021
Patients would participate in therapy sessions to prepare for the use of psychedelics, after which the substances would be administered under the guidance and supervision of trained medical professionals. Right to Try laws permit patients with serious or life-threatening diseases to access drugs that do not yet have government approval.

Burns Levinson (Cannabusiness advisory)
OCTOBER 3, 2018
This marks the first time in history that the DEA has removed any type of cannabis from Schedule I, and clears the way for the sale of the first non-synthetic, cannabis-derived medicine to win federal approval. According to the DEA order, because the drug was recently approved by the FDA, it is now considered to have an accepted medical use.

Cannabis Law Report
AUGUST 18, 2022
Among the first in the nation, Phillips Lytle’s Psychedelics Practice Team combines expertise in pharmaceutical science, FDA regulations and risk management to serve the legal needs of emerging bio-tech sectors. “It also creates new opportunities for researchers, entrepreneurs, investors and pharmaceutical companies.

Cannabis Law Report
JULY 7, 2019
These are many of the common questions and concerns your average patient has with respect to cannabis today and the answer isn’t quite as clear as we would like it to be; however, there is a linguistic trend steering towards the term “medicated” to replace, the pejorative, “high” for cannabis users seeking medical relief. AUTHOR: Anthony S.

SpeedWeed
JULY 23, 2021
Thanks to the new DEA research license, MedPharm will now be able to supply investigational medications across state lines for the purpose of clinical trials. The license only allows MedPharm to conduct research on cannabis that was shifted through certain channels that have been approved by the government and DEA.

SpeedWeed
AUGUST 11, 2021
This trial will be the second of its kind to be conducted so far, and one that is an FDA- and DEA-regulated double-blind, placebo-controlled study. Results of this study showed evidence of the pros and cons to cannabis as a treatment for PTSD patients. The study was funded with $2.2 PTSD affects a large number of military veterans.
The Cannigma
MAY 1, 2023
2 3 As clinical trials around the world proceeded in the late 1960s and 1970s, patients began reporting difficulty tolerating ketamine’s unpleasant hallucinogenic and dissociative properties. It was also after the Vietnam War that widespread ketamine abuse would begin to show up as a party drug.

Veriheal
OCTOBER 23, 2020
House Republicans supported her proposal to defund the DEA and divert those funds to opioid treatment programs. . Patients in Virginia have been waiting and waiting for this day and it’s finally here! Patients in Virginia have been waiting and waiting for this day and it’s finally here! .

Veriheal
OCTOBER 23, 2020
House Republicans supported her proposal to defund the DEA and divert those funds to opioid treatment programs. . Patients in Virginia have been waiting and waiting for this day and it’s finally here! Patients in Virginia have been waiting and waiting for this day and it’s finally here! .

Cannabis Law Report
APRIL 1, 2019
Selling dried cannabis to pharmaceutical companies as well as Cannabidiol (CBD) and Tetrahydrocannabinol (THC) extracts as soon as it is licensed, is the intention of Thailand’s tobacco authority among new measures to turn a profit on its dwindling cigarette business. 49 Percent Foreign Shareholding Allowed. GPO, the Ultra-Modern Drug Maker.

Sensi Seeds
NOVEMBER 28, 2018
The confusion is compounded by the fact another CBD company, GW Pharmaceuticals, the British Biotech and marijuana grower, managed to secure approval from the U.S. CBD Regulation is Needed.

Cannabis Law Report
JULY 30, 2019
The informed patients, cannabis industry employees, and clinicians find it equally difficult to keep updated on Florida’s latest medical marijuana program news and changes. . In light of all this state cannabis information overload, what are some of the reasons a patient ultimately chooses a qualified personal cannabis physician? .

Cannabis Law Report
APRIL 21, 2022
Publication of this process establishes “prior art,” contributing to MAPS’ patient access strategy by making intellectual property public . 30,000 patient doses) of MDMA in a four-step process beginning with a non-controlled starting material. The chemical purity of the final product exceeded 99.9%

SpeedWeed
DECEMBER 28, 2018
Marinol (synthetic THC) has been available by prescription since 1986, and other synthetic cannabinoid drugs are in the works, but Epidiolex is the first plant-derived pharmaceutical to reach the US market. Bummer: Patients Are Still Struggling for Cannabis Access. RELATED STORY.

Canna Law Blog
MAY 7, 2020
What you may not know is that the federal government actually has its own stash of cannabis for limited patient distribution and limited research purposes that it houses at the University of Mississippi (a/k/a) Ole Miss in Oxford, Mississippi. These cannabis products are used by researchers in the U.S.

Canna Law Blog
SEPTEMBER 6, 2023
Many people in the cannabis industry are convinced that this HHS recommendation to the Drug Enforcement Administration (DEA) means that the DEA will undertake this rescheduling (and fairly quickly, too–which would be a huge departure from its refusal to reschedule back in 2016). Just my two cents; feel free to disagree.

Cannabis Law Report
JULY 12, 2019
Side effects were very prevalent in 79% of all patients taking a refined CBD product, some of which were severe like thrombocytopenia and transaminase elevations in the liver. Review: During an open-label trial of pure CBD with Lennox-Gastaut and Dravet patients, 79% of all patients reported side effects.

The Cannigma
JUNE 14, 2021
All that is about to change, thanks to a change at the DEA , which cultivators and industry experts say will be monumental for cannabis research, medical marijuana patients, and potentially the broader legal status of the plant itself. “We BRC) told The Cannigma this week. Junk’ cannabis ‘ill-suited for clinical trials’. Shutterstock).

Canna Law Blog
AUGUST 21, 2020
Although the Second Circuit expressed considerable skepticism of the drug scheduling regime, the court held that before plaintiffs could seek relief in federal court, they must first file a de-scheduling petition with the DEA. At this point, readers may be asking: Why didn’t the plaintiffs file a petition with the DEA? Petition at 3.

Veriheal
AUGUST 31, 2020
Essay Summary: Nishtha wants to further research into THC treatment for patients suffering from neurodegenerative diseases like Alzheimer’s and epilepsy , both of which cause neuron death. Program between St. Bonaventure University and George Washington University School of Medicine. . School: University of Maryland.
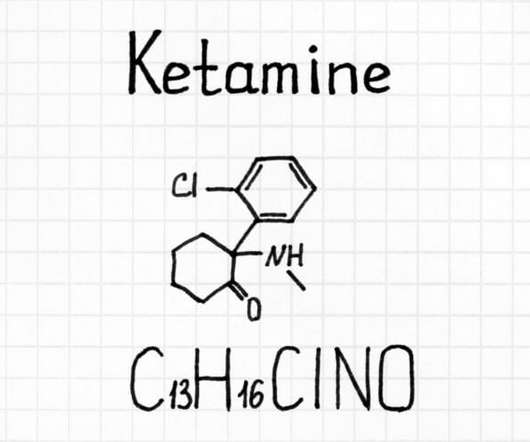
Canna Law Blog
MARCH 4, 2020
People are increasingly using ketamine for ailments that resist treatment through traditional pharmaceutical drugs. “ Off-label use ” (which is extremely common) is: the use of pharmaceutical drugs for an unapproved indication or in an unapproved age group, dosage, or route of administration.

Canna Law Blog
NOVEMBER 16, 2021
The enforcement role moves from DEA to ATF. When it comes to enforcing federal cannabis regulations, the Drug Enforcement Administration (“DEA”) is out and the Bureau of Alcohol, Tobacco, Firearms, and Explosives (“ATF”) is in (in addition to TTB as the primary overseer). . ” SBA fairness.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content