Doctor Sues DEA for Right to Give Psilocybin to Ailing Patients
Project CBD
NOVEMBER 8, 2021
Psilocybin, the psychedelic mushroom extract, has been fast-tracked by the FDA to treat depression, but doctors still can’t use it in their practice.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Project CBD
NOVEMBER 8, 2021
Psilocybin, the psychedelic mushroom extract, has been fast-tracked by the FDA to treat depression, but doctors still can’t use it in their practice.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

MJ Business Attorneys
JUNE 29, 2018
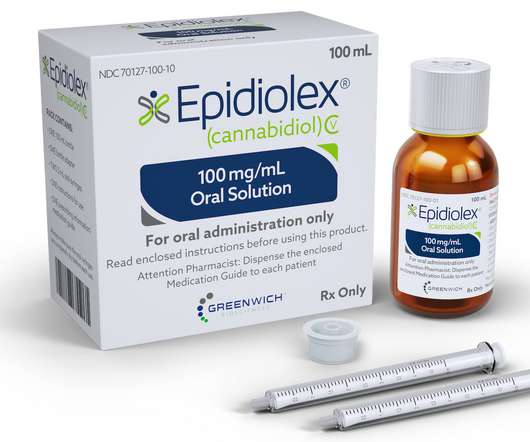
Food and Drug Administration (FDA) approved Epidiolex, a drug containing Cannabidiol (CBD). Epidiolex is the first drug the FDA has approved that contains a substance derived from marijuana, and it is also the first drug approved by the FDA to be used to treat patients with Dravet syndrome. On Monday, the U.S.

NORML
AUGUST 26, 2019
The US Drug Enforcement Administration (DEA) has once again pledged to take action to better facilitate clinical cannabis research. In 2016, the DEA similarly announced the adoption of new rules to expand to supply of research-grade cannabis, but failed to take any further action.

United Patients Group
JANUARY 15, 2023
CBD and THC are two natural substances found in the cannabis plant. Defining CBD and THC. CBD is derived from hemp or marijuana plants, both species of Cannabis Sativa. Hemp-derived CBD must have a THC concentration of 0.3 Hemp-derived CBD must have a THC concentration of 0.3 Psychoactive Effects of CBD and THC.

Project CBD
NOVEMBER 8, 2021
Psilocybin, the psychedelic mushroom extract, has been fast-tracked by the FDA to treat depression, but doctors still can’t use it in their practice.

WeedAdvisor
JULY 4, 2019
Despite mounting evidence into cannabis’ therapeutic benefits, the DEA continues to close its eyes and plug its ears, constantly claiming that there is not enough research to support its medicinal value, according to Marijuana Moment. It could be argued that the DEA is hesitant for the sake of public safety.

Freedom Leaf
OCTOBER 15, 2018
Though it doesn’t get users high like THC-dominant products, CBD is all the rage these days due to its medical benefits and gray-area legality. On September 28, the DEA designated Epidiolex —a plant-based CBD pharmaceutical manufactured by the UK-based GW Pharmaceuticals—a Schedule V drug in the government’s list of controlled substances.

United Patients Group
FEBRUARY 21, 2023
Understanding the 4 Major Differences Between CBD and THC CBD and THC are both compounds that can be found in the Cannabis plant. CBD does not produce any psychoactive effects, while THC does. This means that CBD won’t get you high, while THC will. Hemp must legally have a THC content of 0.3 percent or less.

CannaMD
AUGUST 8, 2020
In honor of National CBD Day, CannaMD wants to clear up some cannabinoid confusion once and for all. So sit back, relax – and if you can’t relax, grab some CBD! What is CBD? Let’s clear up one of the biggest sources of confusion right off the bat: CBD is a cannabis compound called cannabidiol.

Cannabis Law Report
NOVEMBER 22, 2019
MMJ International Holdings, the premier medical cannabis research company, announced that it has received DEA approval to ship THC and CBD from Canada. MMJ International Holdings is developing oral drug products from natural whole plant extract derivatives from the marijuana plant containing THC and CBD. Orphan Drug Designation?

Marijuana Lawyer Blog
JANUARY 12, 2019
As our Los Angeles marijuana patient attorneys can explain, the crux of the argument by plaintiffs of the claim, first filed in 2017, is that the designation ignores the merits of the drug for medicinal purposes. Defendants are acting-Attorney General Matthew Whitaker, the acting director of the DEA and the federal government.

United Patients Group
APRIL 3, 2023
A recommendation from a physician is simply permission (‘recommendation’) for the patient to use cannabis, which can be obtained in any state where medical marijuana is legal. Epidiolex, an FDA-approved medication containing CBD (cannabidiol), has been listed as Schedule 5 by the DEA since June 25th, 2019.

Veriheal
NOVEMBER 6, 2023
For example, there are some states where only CBD-based products are allowed. Others like Idaho, meanwhile, consider even simple CBD possession a crime. In 2015, Idaho state legalized CBD with a THC concentration of less than 3%. Like Idaho, CBD with less than.3% Recreational CBD, meanwhile, must be entirely THC-free.

MedicalJane
JUNE 29, 2018
On June 26, 2018, the FDA granted approval of Epidiolex, a cannabidiol (CBD) oral solution, to GW Research Ltd (GW). The new drug is indicated for the treatment of seizures associated with two rare and severe forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome, in patients two years of age and older.

Burns Levinson (Cannabusiness advisory)
OCTOBER 3, 2018
Last week, the industry was energized by the Drug Enforcement Administration’s order placing certain drugs containing cannabidiol, or CBD, in Schedule V of the Controlled Substances Act. Under the CSA, CBD remains a Schedule I substance, which means that it is not considered to have any currently accepted medical use.

FloridaMarijuana.net
JULY 8, 2018
PTSD patients lack the necessary endocannabinoids to fill the receptor sites properly. Medical marijuana and cannabinoids can be used to treat a patient’s physical and psychological health, alleviating the debilitating symptoms of PTSD. The study has received full approval from the FDA, DEA, and Institutional Review Boards (IRBs).

Veriheal
APRIL 11, 2022
You don’t take that opium poppy and ask a patient to rub it on their lip for morphine. We know that the plant has powerful potential medical effects, but it clearly needs more refinement, more research and more data,” stated Groff, who envisions a future where patients can easily access ailment-targeted cannabis meds.

Veriheal
MAY 6, 2022
Millions of post-traumatic stress disorder (PTSD) patients around the world—such as ex-veterans and military servicemen—tend to relive their traumatic flashbacks and experiences during sleep. Does CBD Impact REM Sleep? Interestingly, research shows that CBD may influence REM sleep, albeit in dose-dependent quantities.

The Joint Blog
MARCH 4, 2021
The United States has had a long, conflicting, relationship with cannabis, cannabidiol(CBD), and other cannabis-derived products. But while some small achievements are being made now, significant changes are going to have to take place for the American CBD industry to reach its full potential as a multi-billion dollar industry.

Cannabis Law Report
MARCH 3, 2022
Former President Bill Clinton has apparently been hearing a lot about the therapeutic potential of CBD products for pain management lately, and, according to a now-deleted media report, said at a recent conference appearance that broad interest in the topic should spur regulators to develop standards for the cannabinoid. Ganjapreneur report.

Weedistry
MARCH 7, 2019
Guzman administered pure THC via a catheter into the tumors of nine hospitalized patients with glioblastoma, who had failed to respond to standard brain-cancer therapies. He postulates that CBD , by virtue of its ability to silence ID -1 expression, could be a breakthrough anti-cancer medication.

Alchimia
APRIL 17, 2018
While further research is needed in both humans and animals, cannabinoids like CBD are being increasingly used to treat diverse symptoms and pains in pets , since many users have realised the great benefits it can provide in some cases. CBD oils and extracts are made from organic hemp crops. Benefits of CBD for pets.

SpeedWeed
MARCH 21, 2021
Though it is naturally occurring in cannabis, Delta-8-THC can be converted in a lab from cannabidiol (CBD) and Delta-9-tetrahydrocannabinol (THC), says Dr. Ethan Russo, M.D., He says he believes it is most commonly converted from CBD. However, he says, “The main source these days seem to be in the lab, turning CBD into it.”

The Blunt Truth
DECEMBER 29, 2023
And now we arrive at the dog days of August , when Congress held a hearing on the Food and Drug Administration’s lack of action on CBD regulation. As of now, this is still a proposal, and it’s anyone’s guess if the DEA (who would do the actual re-scheduling) will think that’s a good idea. But what about federal legislation ??

SpeedWeed
MARCH 17, 2021
Though it is naturally occurring in cannabis, Delta-8-THC can be converted in a lab from cannabidiol (CBD) and Delta-9-tetrahydrocannabinol (THC), says Dr. Ethan Russo, M.D., He says he believes it is most commonly converted from CBD. However, he says, “The main source these days seem to be in the lab, turning CBD into it.”

Cannabis Law Report
MAY 29, 2022
8] Spravato is derived from ketamine and categorized by DEA as a Schedule III controlled substance. To mitigate safety risks, FDA implemented a Risk Evaluation and Mitigation Strategy (REMS) with elements to assure safe use (ETASU) whereby patients self-administer the spray in their healthcare provider’s office under medical supervision.

Veriheal
NOVEMBER 11, 2023
According to the United States Drug Enforcement Administration (DEA), Schedule One drugs are “drugs with no currently accepted medical use and a high potential for abuse.” However, cannabidiol (CBD) and CBD products obtained from hemp plants with less than 0.3% Additionally, seven US states allow the medical use of CBD oil.
Veriheal
DECEMBER 1, 2020
When many people think about cannabis, they think about THC and CBD. An outdated study from the early 1990s conducted on chemotherapy patients that were children showed just like Delta-9 that Delta-8 helped prevent vomiting. The DEA is Already Interfering. Way to go, DEA, on jumping the gun showing your true colors.

SpeedWeed
AUGUST 8, 2021
If all goes according to plan, patients will receive Sativex and the temozolomide chemotherapy drug, temozolomide on its own, or a placebo. In 2018, the CBD and Delta-9-THC oral aerosol spray became the first FDA-approved drug derived from marijuana. That’s how much money it will take to pull the project off. View original article.

Veriheal
DECEMBER 28, 2021
D espite the Drug Enforcement Agency’s (DEA) announcement in May that it would soon start reviewing grower applications for research purposes, cannabis research continues to be tightly restricted. This, researchers say, makes CBD a top choice for relieving the symptoms of neurodegenerative disorders like Alzheimer’s and PTSD.

Cannabis Law Report
NOVEMBER 2, 2021
CBD Oracle commissioned FESA Labs to analyze a total of 51 different delta-8 THC products for cannabinoid levels, with a random subset of 8 being additionally tested for impurities, including heavy metals, solvents, mycotoxins, pesticides and microbial contamination, as well as testing for vitamin E acetate. Photo: CBD Oracle.

LifeCannMD
OCTOBER 15, 2021
Simply put, because Delta 8 is created using hemp-derived CBD it is a legal way of producing the intoxicating effect of THC. In Florida, however, carrying a Medical Marijuana card allows patients to take full advantage of Delta-8. Typically, patients experience an instant feeling of relief.

SpeedWeed
MARCH 21, 2021
Though it is naturally occurring in cannabis, Delta-8-THC can be converted in a lab from cannabidiol (CBD) and Delta-9-tetrahydrocannabinol (THC), says Dr. Ethan Russo, M.D., He says he believes it is most commonly converted from CBD. However, he says, “The main source these days seem to be in the lab, turning CBD into it.”

The Joint Blog
JULY 12, 2019
The increase is in response to the rapidly growing interest in marijuana strains with high levels of THC and CBD. The crop will be divided between high THC and high CBD varieties with “recent interest (in CBD) as a potential medicine for a number of medical conditions,” NIDA said. Others are focused on THC. “We

CannaMD
NOVEMBER 20, 2020
a wealth of new research legitimizing marijuana’s role in patient care – here we are. Making matters worse, there’s only one farm in the entire country with DEA approval to cultivate marijuana for research purposes. For more details, see: CBD May Improve Workplace Performance. Cannabis vs. Opioids.

The Blunt Truth
MARCH 5, 2020
Lawmakers wrestled with the issue of capping THC content in medical marijuana for those patients under the age of 21. The Department of Agriculture delayed a requirement that the DEA conduct all THC testing on hemp crops. To cap or not to cap, that was the question facing the state of Florida this week.

The Joint Blog
AUGUST 5, 2019
The United States Government has agreed to allow for the largest crop of marijuana being grown for research in five years, a direct response to the increased interest in marijuana and its compounds such as THC and CBD. The compound THC causes pot’s mind-altering effect; CBD doesn’t get people high. Others are focused on THC. “We

Veriheal
AUGUST 31, 2020
Essay Summary: Nishtha wants to further research into THC treatment for patients suffering from neurodegenerative diseases like Alzheimer’s and epilepsy , both of which cause neuron death. Program between St. Bonaventure University and George Washington University School of Medicine. .

The Blunt Truth
OCTOBER 3, 2019
They maintain that the limits will put constraints on the amount of marijuana available to patients. In the world of hemp, Senator Mitch McConnell has directed the DEA to figure out how to distinguish hemp from marijuana. ” Since the CBD industry is projected to surpass $20 billion by 2024 , those tests might come in handy.

CannaMD
SEPTEMBER 15, 2020
Current Florida medical marijuana patient? Patients suffering from cancer, epilepsy, chronic seizures, or muscle spasms could use low-THC cannabis products recommended by a licensed doctor. Doctors and patients had to register on the Compassionate Use Registry , an online database maintained by the Florida Department of Health.

Cannabis Law Report
SEPTEMBER 23, 2021
Both notices state most of the delta-8 THC products on the market have been “synthetically converted” from CBD but fail to address the fundamental stakeholder question as to the legality of these products. Delta-8 THC products should be kept out of the reach of children and pets. Should be stored away from children.

Kind Meds (Cannabis Education Blog)
SEPTEMBER 4, 2019
This cautionary tale is premised on the same goal that some dispensary owners, manufacturers, and stockholders are hypervigilant on, to the detriment of hemp patients. received a warning letter from the Federal Drug Administration admonishing them for: Selling CBD-based products online. In a word – greed. How Wall Street Dictates Bud.

NewsMunchies
MAY 20, 2021
This action by the DEA means researchers will be able to study marijuana from more than one grower. By sourcing the plant from more than one facility, scientists can now go out and source materials focused on certain criteria, such as a high THC content, particular terpene profile, or high CBD content. Rate this blog post.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content