Beta-Caryophyllene: Terpene Powerhouse
Project CBD
JUNE 7, 2023
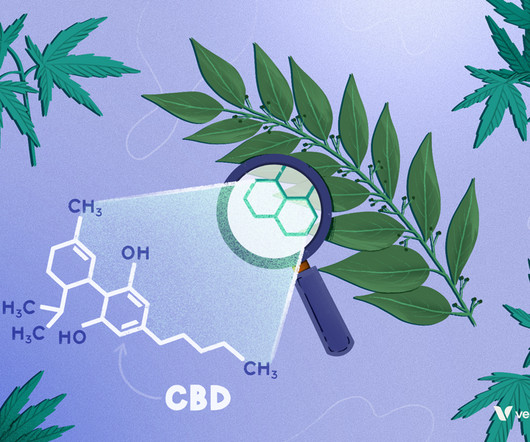
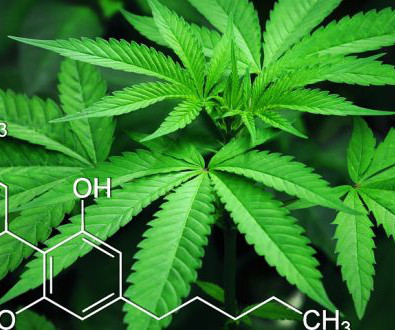
Project CBD recently reported on studies indicating that cannabis terpenes — the compounds that give the plant its robust and distinctive smell — activate the CB1 cannabinoid receptor. What’s more, in the presence of THC (also a CB1 agonist), terpenes appear to modulate cannabinoid activity in varied and interesting ways.
















































Let's personalize your content