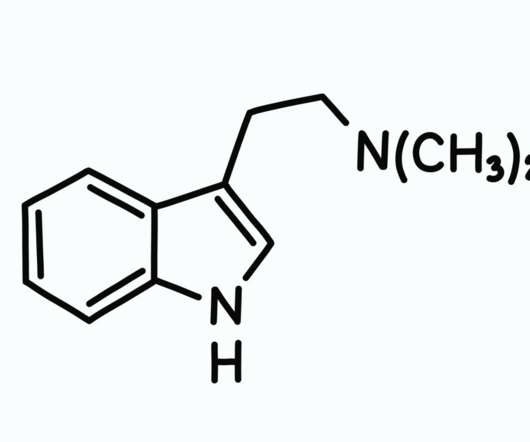
Canada Opens Access for Psilocybin and MDMA Therapy
Cannabis Law Report
FEBRUARY 24, 2022
Subsection 56(1) of the CDSA will exempt medical providers from enforcement of the CDSA when providing psilocybin and MDMA treatment to patients suffering serious or terminal health conditions. In 2013, section C and J of Canada’s FDR statute were amended so that “restricted drugs” were inaccessible through SAP.








































Let's personalize your content