DMT Therapy: Coming to a Clinic Near You?
Canna Law Blog
NOVEMBER 27, 2021
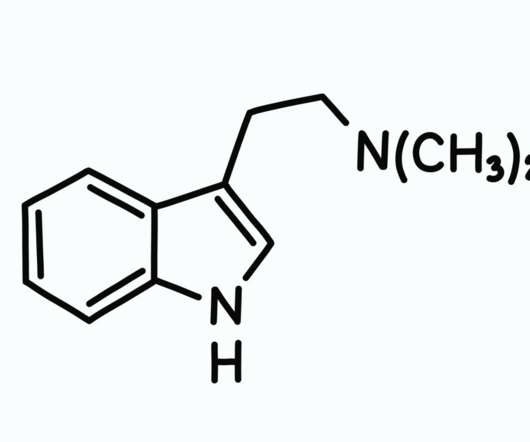
So according to the DEA, its abuse potential is high and it has no medical use. Also, according to the DEA, “the history of human experience probably goes back several hundred years since DMT usage is associated with a number of religious practices and rituals.” MDMA (Ecstasy) Nears FDA Approval for PTSD Treatment.
























Let's personalize your content