DEA Faces Lawsuit For Denying End of Life Patients the Right to Try Psychedelics Therapy
Veriheal
JUNE 29, 2021
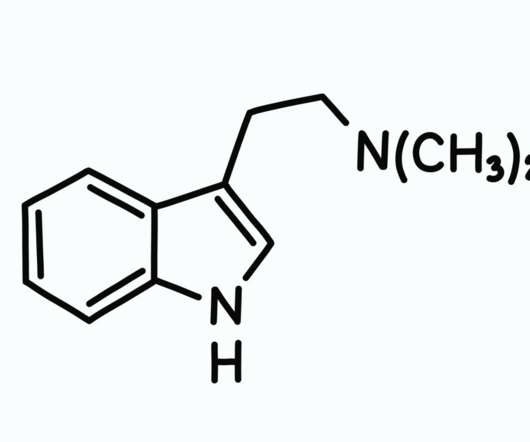
In the last 100 years, many of the plant therapies that were embraced in history were outlawed by governments around the world and replaced by lab-made synthetic drugs we label medicine. The FDA refers to this program as the expanded access program on their website. Cannabinoid Therapies for Cancer Patients.


















Let's personalize your content