The Cannigma has always taken a data-informed approach to content creation – though up until recently we’ve been primarily leaning on the existing peer-reviewed literature to guide our content.
This approach has served us well and contributed to the creation of oodles of informative content that helps you, along with millions of others, to make informed decisions about cannabis. But, it’s not enough.
We need more data – human data – on specific applications for the plant – symptoms, use cases, conditions and patient types. We need more data on “minor” cannabinoids, on terpenes, on specific cannabinoid ratios and formulations. On hemp-derived and synthetic cannabinoids alike. The list goes on.
Now, there are some major barriers here; clinical research is expensive, and slow – and for good reason. The medical community insists on long-term data to safeguard us from products with dangerous side effects that don’t necessarily show up on first use. But cannabis clearly does not fall into this category – humans have been using it as a medicine for thousands of years and there’s not been a single reported death.
If we waited for enough evidence for each individual indication, delivery method and combination of cannabinoids and terpenes, for example, we’d be unlikely to see any cannabis products on shelves for decades, the costs would be outrageous – and patients would be suffering in the meantime.
Gathering large consumer data sets and interpreting them appropriately can help us make the learning faster and cheaper. And in the cannabis space specifically, we have a real opportunity. Whether we like it or not, whether it’s legal or not, people are using weed all the time. Estimates suggest there are over 200 million of them, in fact. If we can tap into even a small percentage of that consumer data – there’s a lot we can learn.
To be sure, it’s not just the cannabis industry that’s going this way – it’s the entire health ecosystem. Scientists, doctors, regulators and product development companies alike have been working towards coming to a consensus on how to analyze and draw clinical conclusions from novel data sources (i.e. not traditional clinical data) for years.
This is also why Google’s life sciences research organization, Verily Health Sciences, is working closely with the FDA to develop frameworks to optimize the analysis of real-world data (RWD) to create real-world evidence – which can complement clinical data and get us learning more, faster, and cheaper. According to Verily’s Chief Medical Officer, Amy Abernathyy, patient-centric software solutions that gather large-scale data are key. 1
So we need real-world data. One-off anecdotal reports are useful too, but they can be inconsistent in format and focus; they are not repeatable methods or measurements. Analyzing data from thousands of cannabis consumers using commercially-available products and leveraging validated measurement methods brings us closer to understanding which products are most likely to help and in what situations. So that’s what we’re setting out to do.
Collecting cannabis data from The Cannigma audience
In June, 2023, we launched a community research effort with data and software company MoreBetter, creators of the Releaf App. Since then, we’ve opened a total of five data collection efforts, in which we invite our audience to try products from partner brands, and share their experiences during a 4-week engagement.
After that, the data team crunches the numbers to produce an insights report for each product, which participating brands can turn into data-based marketing collateral (here’s an awesome example). And then with this data, we create content that’s not only reviewed by one of our scientific experts, but also based on RWD, directly from our audience (and here’s an example of that).
In other words – we get to support content and marketing that actually means something; real-world insights garnered from data over time rather than one-off reviews and questionnaires.
How real-world data differs from traditional research findings
So what’s the difference between this data collection effort and a formal scientific experiment (or study)? There are a few specific differences that are important to note:
- Real-world data collection. Participants stay in their homes, going about their daily lives, and reporting their experiences when prompted by a daily text message. No labs, no wires, no clipboards – just real, daily life.
- Open label: Participants know what brand/product they’re trying, along with the dosage.
- Not a true scientific experiment: There’s no control group with which to compare and contextualize results.
Real-world data collection has advantages and disadvantages over a formal scientific study. The fact that participants can report how the product affects their daily lives, rather than an isolated screenshot in a lab, is the main benefit. And then on the other hand, the fact that there’s no control group is the main downside.
The placebo effect is real. So the downside of a data collection project like this is that there is no correction, no control group, no stabilizing factor. In other words, there’s no clear way to separate causation and correlation, and to tell if the results are directly related to the product in question, or simply the fact that people started taking this product with the intent of sleeping better, for example, so then their sleep reports improved after taking it.
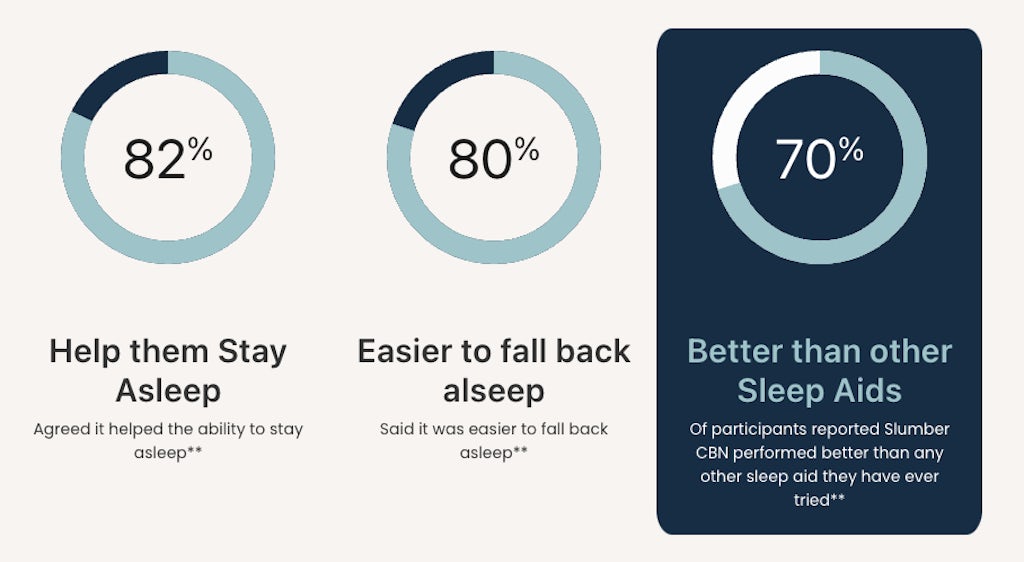
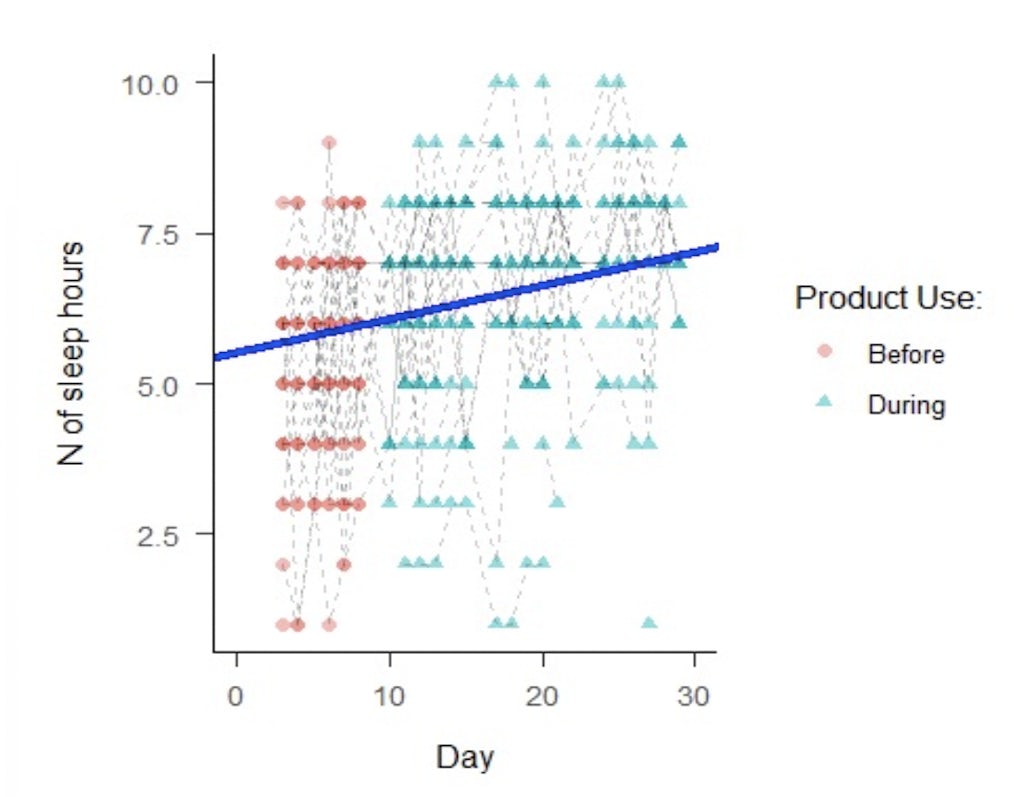
But we do have some checks and balances in place. Participants answer questions for a full week before they start trying the product, in order to establish a baseline assessment. So for example in a recent campaign on sleep, we were able to establish the average daily hours of sleep at the beginning of the engagement, and compare it to the results after three weeks of product use.
The future of cannabis data
With so many products and use cases to test, as we talked about earlier, this far-from-perfect system gets us a damn sight closer to answers than anything else will. The challenge for us as an organization, and as an industry, is going to be interpreting the data in a way that makes it comparable in value to traditional clinical evidence. In the words of Abernathy, “In a sense, we will know we have succeeded in this task at the point it will seem unusual to distinguish the category of ‘real-world’ data at all — it will just be considered ‘data’.”
So far on The Cannigma, we’ve run campaigns on sleep, appetite control and different types of chronic pain, and upcoming topics include focus, PMS and arthritis – what happens next is up to all of us, brands, consumers and media outlets alike.
Sources
- Abernethy A. (2023). Time for real-world health data to become routine. Nature medicine, 29(6), 1317. https://doi.org/10.1038/s41591-023-02337-0
Sign up for bi-weekly updates, packed full of cannabis education, recipes, and tips. Your inbox will love it.

 Shop
Shop Support
Support



















