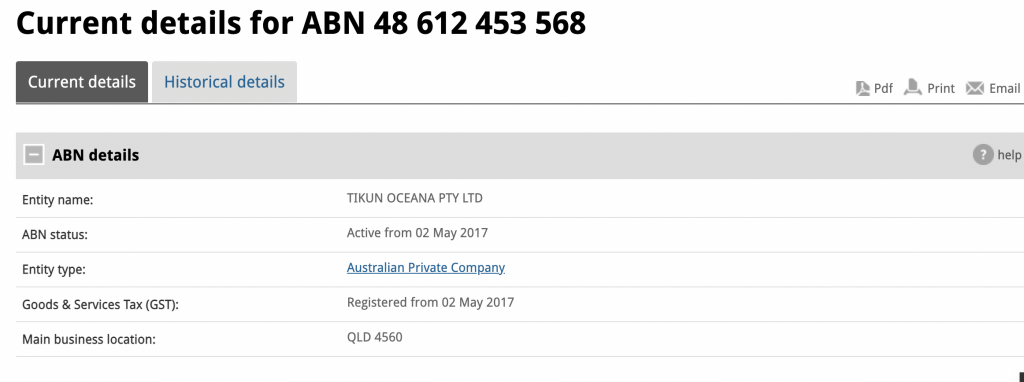
Sunshine Coast based Tikun Oceana, formerly known as Medifarm, is now under the control of administrators from Cor Cordis.The company was founded ……..
Predictably Murdoch media has this behind a paywall if you want to pay to learn more
All we can tell you is that as recently as the 8th of this month their media page implies everything is tickedy boo.
As yet no announcement on the site that administrators have been called in
Katy Williams Day makes no mention of the company at all on her Linked In page
The company linked in page is equally uniformative
The ABC reported back in 2018
The first Australian company to be licensed to cultivate and manufacture medicinal marijuana has praised the Federal Government’s plan to allow exports of cannabis-based medicines.
Privately-owned and Queensland-based Medifarm is close to completing construction of a secure facility in a secret location on the Sunshine Coast.
Medifarm founder, Adam Benjamin welcomed Health Minister Greg Hunt’s ‘excellent’ export announcement.
“We consider the news another great step forward.”
Medifarm has an exclusive international intellectual property partnership with Israeli based company, Tikun Olam, which pioneered world production 12 years ago.
Tikun Olam will provide the mother stock for Medifarm’s medicinal marijuana plants.
“We’re hoping to have these first genetic materials landed within a month and local production out the farm gate within the next three to four months,” Mr Benjamin said.
“We were consulted by the Office of Drug Control, the federal licensing authority, last year about whether we were interested in exporting.
Read more at
Alarm bells should have rung at this point
ABC report 9 January 2018
Secrecy has surrounded a media visit to Queensland’s first licensed cannabis farm, which is nearing completion on the Sunshine Coast.
The first medicinal cannabis ‘mother’ plants are expected to be imported from Israel within a month, with manufacturing scheduled to begin within the first half of this year, to supply 5,000 Australian patients.
Journalists were required to sign non-disclosure agreements, promising not to reveal the location of the multi-million dollar facility, before Medifarm director Adam Benjamin led a convoy to the high-security site.
At the end of a rural road, high security fences topped with barbed wire surrounded a large greenhouse that reporters were not allowed to enter.
“I guess one of the reasons the licensing authority, the Office of Drug Control through federal health, liked what we proposed and awarded our licences is everything’s co-located,” Mr Benjamin said.
“The licensing authority has the right to knock on our doors any time of the day they like and come and inspect, and the buck stops with us.”
Biometric scanning for workers
When workers arrive at the compound they will be identified by fingerprint scanning, and enter through a steel turnstile.
“They get changed. They then go off to their separate sections, which are again all controlled by biometric fingerprint scanning for access,” Mr Benjamin said.
Federal Member for Fairfax Ted O’Brien said strict controls of the supply chain would be critical to the Government’s aspirations to make Australia the number one world player in medicinal cannabis.
Last week, federal Health Minister Greg Hunt announced changes to regulations that would allow for medicinal cannabis exports, capitalising on what the Government estimated would be a $70 billion industry by 2025.
“We need to get approvals. The Global Narcotics Control Board has given a big tick to the framework that’s being adopted in Australia,” Mr O’Brien said.
“That can only stay if we have the players who manufacture this product doing exactly what Medifarm is doing.
“It’s about building a new industry and that’s why it’s so critical that this company and others comply.
“A lot of the countries that are currently active are exploring the opportunity of imports but also exports themselves. You’ve got multiple states, particularly in the US that are very active. Canada’s coming on stream.”
Different strains for different conditions
Medifarm is one of just four licensed medicinal cannabis farms to gain approval in Australia.
The company has an exclusive international intellectual property partnership with Israeli-based company Tikun Olam, which pioneered world production 12 years ago.
Different strains are grown to treat different medical conditions.
The schedule eight pharmaceutical medicine has gained federal approval to treat conditions including cancer-related pain, nausea and vomiting, multiple sclerosis and epilepsy, and for use in palliative care.
But bottlenecks remain to doctors prescribing the controversial treatment.
“Everything will be quality tested onsite. We will then validate it off site at the University of the Sunshine Coast,” Mr Benjamin said.
Two large steel vaults have been craned onto the site. One will be used to store the harvested, dried raw product.
“It’s a very heavy steel safe door, and then when it’s needed for extraction purposes it goes through to the laboratory, becomes oil, gets tested and then all the finished product goes off into the second vault door,” Mr Benjamin said.
“It’s fire-proofed to what’s called 90-90, which means you can have a blazing inferno outside and nothing inside will get touched for 90 minutes at the very least, which obviously gives you more than enough time to work on anything that may have happened.
“Not just the perimeter security, but also the safety and security onsite is paramount.”
And guess who was at the official opening, yep, former health minister Greg Hunt and local member Ted O’Brian, now shadow minister for the enviroment (not a joke!)
Thanks to the Ministry for Health & Aged Care we get a full transcript of toadying.. Here it is
Doorstop interview at Medifarm facility on the Sunshine Coast
Read the transcript of Minister Hunt speaking about medicinal use of cannabis at the opening of the Medifarm facility on the Sunshine Coast.

The Hon Greg Hunt MP
Former Minister for Health and Aged Care
ADAM BENJAMIN:
Ladies and gentlemen, welcome.
Thank you all very much for being here today. We’re in the business of helping patients, we’re in the business of helping Aussie patients and we’re in a privileged moment in time where we now must re-introduce medical cannabis to mainstream medicine just like it used to be prior to 1937.
We are at the crossroads of good agriculture meets good health care which meets great policy.
And we thank the Federal Health Minister for being here for officially opening us. We thank the Member for Fairfax Ted O’Brien for his unflappable support, amazing, right from the start and his vision and I’d like to thank my wonderful wife for her fabulous support and my business partner Mordecai Cohen for his vision and friendship.
So again, we are here because we are helping the patients.
I would like to dedicate today to a wonderful boy, Sam Martin, who passed away in 2014 and his family and their story is the inspiration for what we’re doing here today. So we now have a duty of care to help the Aussie patient and that’s what we’re here to do. Thank you.
TED O’BRIEN:
Adam. Thank you very much, Adam, and a big congratulations to you, to Mordecai, wonderful to see your families here today, it really is.
And also acknowledging Ed and the broader team here at Medifarm and how delighted we are to be here particularly with the Health Minister we know that the Sunshine Coast is probably the most entrepreneurial region in Australia.
And here we have a site that gets opened today that does bring together the best of Australia. We know we are world leaders when it comes to healthcare, we’re world leaders when it comes to medicines and here we are also as world leaders when it comes to agriculture.
And when you have agriculture and health come together with strong robust government policy, we have new businesses emerge using the best intellectual property that the world has to offer.
And so delighted to be here today a very big congratulations and with that, please welcome the Health Minister Greg Hunt.
GREG HUNT:
Thanks very much, thanks very much to O’Brien, to Adam, to you and your family, to Mordecai, to you and your family. [Speaks in Hebrew] So my Hebrew is not as strong as it once was but to everybody associated with Medifarm, it’s a privilege to be here today because of two things.
One is you are creating something new which is about opportunity and health outcomes for patients and you’re helping secondly, to contribute to the Australian economy and growth.
And just to walk around this high quality, high security facility means that you come across Australians who are involved, people from France, people from Israel, people from Germany.
So it’s a multinational workforce and it’s an international centre of patient care, that’s the important thing.
So medicinal cannabis is something which this Government has legalised subject to the most rigorous conditions and arguably some of the most rigorous conditions in the world which is why Europe looks to Australia as a preferred supplier.
We not only legalised it but we then made sure that there was a contingency reserve, it was one of the first decisions I made on coming into this office, available for patients as production was being developed.
Beyond that, we then opened up the export market and we did that so as to ensure that there was both priority for Australian patients, that’s a prerequisite of the export market, but also the capacity for Australian firms to provide health care to the world.
And interestingly, because of a confluence of good policy and good practice Australia, now has an almost unique opportunity in Europe with enormous growth potential for Australian firms at this point.
That brings me to Medifarm and Medifarm at the moment is producing about 1.2 tonnes to supply up to 3000 patients a year in Australia of product. I think the plan, Mordecai, is to grow that to about seven tonnes a year on this current site and it is a world leading facility.
And this is about helping patients who might have epilepsy, who might be suffering from cancer, post-traumatic stress or in the palliative phases of life.
And so it’s about ensuring that there is the best care available. It is a highly regulated sector and we make no apologies for that. But most importantly, it’s about ensuring that if the doctors determine that the medicine should be available, it can be available and to have a pioneer such as Medifarm, they have three licences all up in Australia.
We’ve now issued, the latest advice I have, 81 licences of just over 12,000 patients and scripts have been issued, so patients benefiting from over 12,000 scripts and Medifarm has three of the 81 licences.
And that’s an immensely important step forward. So it takes a lot of courage to invest, to create, and to bring it all together, but it looks as if what we’re going to see is better outcomes for Australian patients in the most regulated and safe environment that we could possibly establish.
And that’s a tremendous outcome for the Sunshine Coast, for Queensland, for Australia, but above all else, for the patients. So I am delighted to officially declare open Medifarm’s Queensland operations.
Happy to take any questions either on Medifarm or other issues after that.
JOURNALIST:
So Minister there was actually …
JOURNALIST:
[Inaudible] one of the holdups, there isn’t actually medicine coming out of this plant yet. I understand the holdup is some regulatory paperwork at a federal level. Why can’t you hasten things up a bit?
GREG HUNT:
So the Office of Drug Control has responsibility for that, and they are very, very assiduous. And one of the things that everybody would want is for us to make sure that we are operating in an efficient way, but in a safe way.
We introduced a one-stop shop with the state’s, New South Wales was first and Queensland was a little bit slower than some of the other states, and that is one of the issues. But we now have the states and territories on board.
JOURNALIST:
So when will patients actually have these products in their hands? The end of last year we were told that it would be ready in the first quarter of this year. How far off are patients from having that?
ADAM BENJAMIN:
If I may add to that, and I believe Australia’s not only got the right legislation here, but the best legislation and the best quality. So from our perspective, you asked when will our product be available.
We’ve already completed to harvests, so two growth cycles of the four- the five that can go on in a year.
Very important if we are here to help the Aussie patient that we have a continuity of supply. We must build up our reserves, which we’ve done.
We didn’t take the media through today, but we have a huge amount that we’re building up so that as we come to market, it’s very important that that supply chain is continuous and the quality is continuous.
JOURNALIST:
So is that this year? Patients having those products?
ADAM BENJAMIN:
Absolutely. Absolutely.
GREG HUNT:
So there you go. So that’s a business decision. I think a sensible one in terms of managing their flows of they have patient continuity.
JOURNALIST:
So Minister related to that, there was some confusion about what today sort of represents. There was the whether it’s an Australian first, a Queensland first. What’s today?
GREG HUNT:
So today is the opening of Medifarm, and Medifarm is one of the leading providers in Australia.
And as I said, we’ve worked very hard. We’ve produced 81 licences for either production, manufacture, or research, and Medifarm is right at the national forefront. But what is today? It is the official opening of Medifarm.
JOURNALIST:
Is Medifarm, this facility, is this the first facility in Australia or Queensland to be producing medicinal cannabis? Or where do we stand with that?
GREG HUNT:
So where we’re at the moment is you’ve got a number of facilities around the country that are in different stages of production that have been fully licensed. So it’s up to each of those to indicate their own. But we have fulfilled over 12,500 prescriptions in Australia.
JOURNALIST:
And these products…
JOURNALIST:
Using Australian product or is that imported product?
GREG HUNT:
So what we did was we opened the market for the import, and there is no barrier now to Australian product, and that will be determined by each supply with the TGA.
JOURNALIST:
Is there anyone using an Australian product yet?
GREG HUNT:
So we’re ready to roll. So when would you say is your first Australian product?
ADAM BENJAMIN:
Ours will be ready before the end of the year. If I can add to your question, sorry. In terms of imported product, obviously we are a proud, Australian local company.
We are not currently importing products, but I do applaud the Government for allowing a stop-gap measure. We know there are hundreds of thousands of Aussie patients in need, and to help that stop-gap measure before local product becomes available, before Medifarm helps Aussie patients before the end of this year, that stop-gap measure has to come from overseas.
So we’ll be proud to contribute as soon as we can.
JOURNALIST:
So when medicines start coming out of this facility, that will be the first Australian produced medicinal cannabis going to patients, is that?
ADAM BENJAMIN:
There are other facilities. I don’t know about the competitors out there. I know that Medifarm was the first fully licensed, so cultivation and manufacturing and research licensed company in Australia. Yes, we are a proud fore-frontrunner.
As for the other 81 licences or the other 78 licences, good on them. People often ask, don’t you find this competitive? If you’ve got to win the race to help the Aussie patient, what an amazing race.
JOURNALIST:
These products can now be exported internationally as well, is that correct? Once they’re ready?
GREG HUNT:
So we’ve opened up the export pathway and then it’s up to each individual firm. And we already have Australian firms that have export licenses.
JOURNALIST:
Minister, you might be in a better position to answer that question. In terms of are there- is there Australian produced medicinal cannabis in the market and going to patients currently?
GREG HUNT:
My expectation is that the first of that will occur within the coming months.
JOURNALIST:
And that will be from this facility?
GREG HUNT:
Well it’ll be an interesting- it will be a race between them. I know that there’s healthy competition to be the first. I would say Medifarm is very well placed to be that first.
JOURNALIST:
Minister, now that a lot of these farms are starting to come online, the product will soon be available. One of the main complaints from prescribing doctors is that the paperwork is simply too complicated, and many are saying they just don’t want to do it.
Is the Government going to look at making that bit a bit easier for doctors?
GREG HUNT:
I think that was a legitimate complaint which is why we moved to establish one-stop shop agreements with the states and territories. Queensland is part of that. New South Wales was the first.
We now have a turnaround time of less than 48 hours between the prescription and the fulfilment.
JOURNALIST:
And how expensive is it for patients?
GREG HUNT:
It will depend for each patient. I’ll let Adam talk about the price for his own product.
ADAM BENJAMIN:
Sure. Look that’s a wonderful lead in. However due to the TGA, the Therapeutic Goods Administration, which is Australians quality control of medicine, which happens to be a world leader right up there with FDA, we do not advertise our product.
So price and efficacy is left up to the doctor as it should be. We all at some point in time have been to a doctor and it’s their clinical judgment that counts. I guess what we always remember is the power is with the patient in this industry because it’s their health that counts most.
JOURNALIST:
Adam, you talked there about the patients. People will be watching this thinking that I perhaps may have a- something that could be treated. How would a patient go about talking to their GP, about access to this kind of medicine?
ADAM BENJAMIN:
So, exactly as you say, talk to your GP. Although this is a bit of a James Bond industry, if you strip away the words medical cannabis and you say schedule 8 prescription medicine, most of us know you go to your doctor.
If you’re the right patient, you get the right product. It’s dispensed through a secure pharmacy. If your doctor needs more information, of course please visit Medifarm and we’re happy to assist.
…/…
JOURNALIST:
Adam, it was mentioned inside that those plants that you- that we were standing in front of would help two patients per year. How far off are they from being able to be sent away and sold to the [inaudible]?
ADAM BENJAMIN:
Sure, so yes. In terms of the statistics, every plant that we saw today will produce enough medicine for two Aussie patients for a year’s supply. As mentioned before we’ve done two harvests. We have a significant volume in the vault. What you see today is a turnkey solution.
We are fully licensed to go from plant to patient with those medicines from this facility and we plan to do that before the end of this year.
JOURNALIST:
Those ones though, how far off are they that we just saw?
ADAM BENJAMIN:
Those plants?
JOURNALIST:
Yeah.
ADAM BENJAMIN:
Those plants will probably have another 40 days’ growth.
JOURNALIST:
And the bottling process and everything like that, is that all, when you say you’ve got stockpiles, that’s all ready to go?
ADAM BENJAMIN:
Yes, it is. So the harvests that occur are in the vault. But look we’re blessed, we’re blessed to live in Australia. We have wonderful climate and this is the merging of good agriculture meeting good healthcare.
JOURNALIST:
And Adam just quickly, your medicines, what are the types of ailments that your product will treated?
ADAM BENJAMIN:
Perhaps if I can just quickly talk about our exclusivity with the Israeli partnership, Tikkun Olam, Tikkun Olam being the global pioneers important for us to help Aussies as quickly as possible.
They have purpose bred genetics, purpose bred genetics for medical conditions such as the Minister mentioned; cancer, post-traumatic stress disorder, palliative care, improving the quality of life, spasticity such as multiple sclerosis.
So without going on to the advertising side of things, very important to have your clinical trials obviously based on products. Most important to have your clinical trials based on the genetics of your plants.
DOCUMENTS
download download downloadAs they say, when we know more we’ll tell you