Though cannabis is becoming increasingly legal across the nation, both for recreational and medical purposes, there’s no sign that the US government intends to legalize cannabis on the federal level. This causes complications as more people than ever before are exploring cannabis for medical purposes. The lack of federal legalization limits the way federally funded organizations are able to interact with cannabis. It curtails the amounts and types of research that are able to be done, placing unfortunate limitations on how much we can learn about the uses of cannabis—and without extensive research, of course, it’s that much more difficult to fight for federal legalization.
A lack of federal legalization also makes it more difficult for the Food and Drug Administration (FDA) to approve cannabis-based products. Whether for medical or recreational purposes, most of the cannabis products we are currently able to procure have not been FDA approved yet. To date, the organization has no application for approval of any cannabis-based medical product.
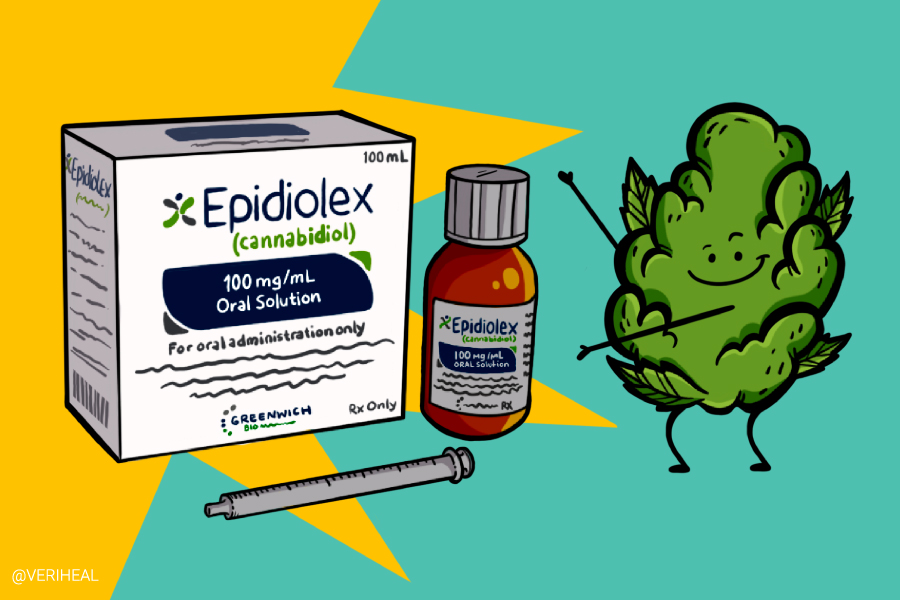
However, there is one notable example—Epidiolex. And recently, the FDA has added to its accepted use of the drug.
What is Epidiolex?
Epidiolex is the sole cannabis-derived pharmaceutical that has been approved by the FDA – although it is worth noting that the organization has also approved three synthetic cannabis-related products. Epidiolex has a complex history. It was approved in 2018 as a Schedule V substance, which is to say, a substance with a low potential for abuse considered not to be dangerous. However, Epidiolex contains CBD. Up until March 2020, CBD was lumped in with cannabis as a Schedule I substance—highly addictive and with no accepted medical uses.
Epidiolex was approved specifically for treating patients suffering from seizures associated with Dravet Syndrome, which is a severe form of epilepsy that affects between 20,000 and 40,000 people worldwide. Though Dravet Syndrome typically manifests itself in the first year of life, Epidiolex is only approved for treating patients two years of age and older.
Why You Should Get Your Medical Marijuana Card
Veriheal has satisfied millions of patients nationwide by giving them access to these benefits
- Larger purchase limits
- Peace of mind
- Enhanced legal protection
- Access to higher potency strains
- Save up to 25% on cannabis purchases
- Skip the line at the dispensary
Of course, it’s wonderful to see the FDA approve this substance to help those suffering from this rare condition. But we know that cannabis is effective at helping to manage seizures caused by other forms of epilepsy—as well as other medical conditions—also. So why limit the use of Epidiolex to just this one condition?
The FDA Adds a New Condition for Treatment
It seems the FDA has been thinking along these same lines.
Just this year, Epidiolex was approved for the treatment of Tuberous Sclerosis Complex, a condition characterized by the growth of benign tumors throughout the body. Though these tumors are noncancerous in nature, their presence can cause health problems, including epilepsy. Tuberous sclerosis complex affects about a million people worldwide. About 85% of these patients experience epilepsy as an effect of the condition, and more than half are unable to achieve seizure control.
However, trial results have shown a positive impact when patients were treated with Epidiolex, and doctors are pleased to add this new option to their list of potential tools for helping their patients, Epidiolex could make a difference in the lives of people suffering from tuberous sclerosis complex, and that’s a huge step for the FDA to take.
It also represents forward motion in the way we deal with cannabis in this country. Though the lack of federal legalization can be frustrating and difficult, and though it is holding us back, it is always encouraging to see organizations like the FDA make movements in the right direction. Soon, we hope to see cannabis-based treatment options made available for people managing a much wider variety of health conditions.
Author, Share & Comments